Flocculants: How Do They Work and Why?
This is a guest post by Bill Hancock, President of Zeroday Enterprises
How Do Flocculants Work and Why?
Have you ever wondered how flocculants work at the colloid (small particle) level?
Flocculants enhance, and are often required to make possible solid-liquid separations from turbid to high percent solids in water. Typically, solid particle sizes in water have a wide normal distribution and are inorganic and organic based. If these particles are denser than water, the particles will settle to the bottom of a container if given sufficient time; however, many of the smaller, lighter particles remain suspended (think a cloudy mud puddle that appears to swirl with activity) for a much longer time than allowed with a typical residence time. That is because the particles, or colloids, are small enough to remain suspended by external forces including Brownian motion (interaction with the water molecules), thermal currents, dispersive surface charges and the like. These are the hardest particles to treat because they are so fine and do not easily and quickly settle.
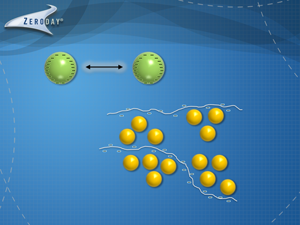
Unless the particles are uniformly coarse (depending on the water chemistry conditions and relative solid and water densities, coarse particles might be considered greater than 100 mesh or greater than 210 microns) and rapidly settle by gravity, flocculants are required to aggregate multiple particles together as ‘floccules’ which are pseudo-large particles. Enter the flocculants!
Polymers are ubiquitous materials ranging from nylon, polyethylene plastics, Teflon, and starches to amino acids. Flocculants belong to the water soluble polymer class, and so they fully dissolve in water. These are acrylamide based with functionality groups which allow the polymers to readily chemically adsorb to particles. These polymers are very long (for perspective, if you expanded a flocculant molecule to 1 inch in diameter, the total length would be on the order of 1.25 miles long!) As flocculant molecules dissolve in water, these molecular chains (ropes) are free to uncoil and expand, but are never completely linear due to random Brownian motion and water thermal current effects.
In effect, these flocculant ropes lasso aggregates of particles together. Since the polymer chains are very long, these polymers agglomerate multiple colloidal and coarse particles together. As these flocculated aggregates continue to mix, the polymer rope continues pulling the particle aggregate into a tighter and denser floccule, which causes the particles to settle more readily. The larger floccules are more easily filtered, centrifuged and floated in a dissolved air flotation unit.
A simple visual demonstration of this process is represented in the accompanying animation video. The animation shows the initial step of ‘coagulation’ where a short cationic (positive) charged polymer coagulant is added to partially neutralize the repulsive particle negative charges and induces pin flocc aggregation of the colloidal particles. Then the flocculant molecules ‘lasso’ and flocculate these pin floccs into larger floccules.
Bill Hancock is an internationally recognized expert in mineral processing technologies, technical marketing management and water treatment. Hancock founded and owns Zeroday Enterprises which supplies chemical mix-feed systems, LTM conductive slurry level monitoring probes, peristaltic hose and tube pumps, mixers and flocculant and coagulant chemicals. He also founded Argo Consulting—a technical and technical marketing consulting practice focused on providing mineral processing, water treatment and technical marketing consulting services to the mining industry.

